PANCREAZE is indicated for the treatment of exocrine pancreatic insufficiency due to cystic fibrosis or other conditions.
Fibrosing colonopathy is associated with high-dose use of pancreatic enzyme replacement. Exercise caution when doses of PANCREAZE (pancrelipase) exceed 2,500 lipase units/kg body weight per meal (or greater than 10,000 lipase units/kg body weight per day).
Hyperuricemia may develop. Consider monitoring uric acid levels in patients with hyperuricemia, gout, or renal impairment.
To avoid irritation of oral mucosa, do not chew PANCREAZE or retain in the mouth.
There is theoretical risk of viral transmission with all pancreatic enzyme products including PANCREAZE. Although it has never been reported, it may be possible for a person to get a viral infection from taking pancreatic enzyme products that come from pigs.
Exercise caution when administering pancrelipase to a patient with a known allergy to proteins of porcine origin.
Most common adverse reactions are: abdominal pain, flatulence, diarrhea, abnormal feces, and fatigue.
PANCREAZE is not interchangeable with any other pancrelipase products.
Dosing should not exceed the recommended maximum dosage set forth by the Cystic Fibrosis Foundation Consensus Conferences Guidelines.
Please read the PANCREAZE Medication Guide and PANCREAZE Product Information.
References:
1. PANCREAZE Full Prescribing Information. Campbell, CA: VIVUS LLC; 2022.
2. Trapnell BC, et al. Efficacy and safety of PANCREAZE for treatment of exocrine pancreatic insufficiency due to cystic fibrosis. J Cyst Fibros. 011;10(5):350-356.
3. Fieker A, Philpott J, Armand M. Enzyme replacement therapy for pancreatic insufficiency: present and future. Clin Exp Gastroenterol. 2011;4:55-73.
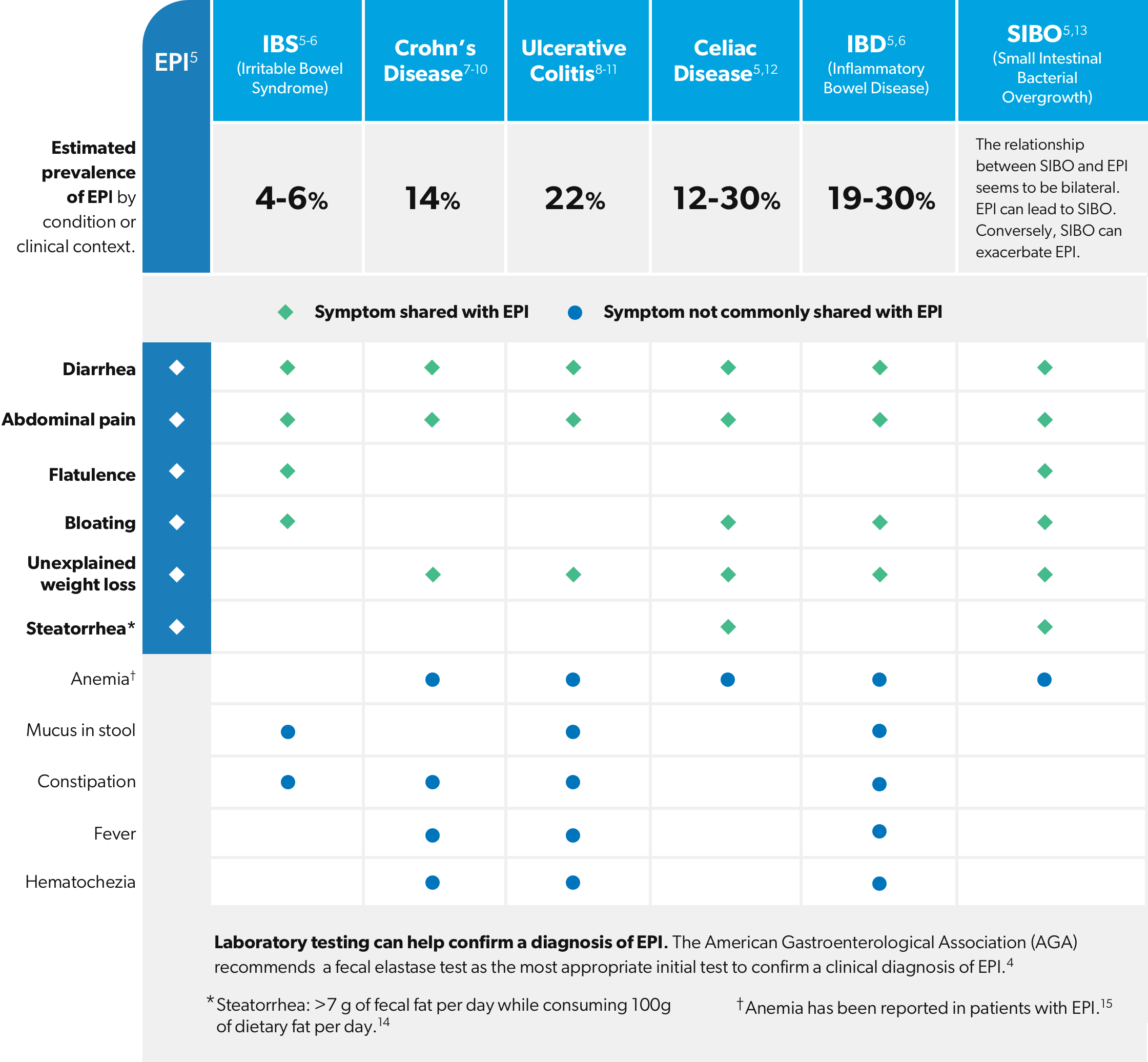
4. Whitcomb, DC, Buchner, AM, Forsmark, CE. AGA Clinical Practice Update on the Epidemiology, Evaluation, and Management of Exocrine Pancreatic Insufficiency: Expert Review. Gastroenterology. 2023;165:1292–1301.
5. Othman MO, Harb D, Barkin JA. Introduction and practical approach to exocrine pancreatic insufficiency for the practicing clinician. Int J Clin Pract. 2018;72:e13066.
6. Crohns and Colitis Foundation (n.d). IBS vs IBD. Retrieved from https:/www.crohnscolitisfoundation.org/what-is-ibd/ibs-vs-ibd
7. Crohns and Colitis Foundation (n.d). Signs and Symptoms of Crohn’s Disease. Retrieved from https://www.crohnscolitisfoundation.org/what-is-crohns-disease/symptoms
8. Kaitha S, Bashir M, Ali T. Iron deficiency anemia in inflammatory bowel disease. World J Gastrointest Pathophysiol. 2015 August 15; 6(3): 62-72.
9. Perler et al. Presenting symptoms in inflammatory bowel disease: descriptive analysis of a community-based inception cohort. BMC Gastroenterology (2019); 19:47.
10. Fousekis FS, Theopistos VI, Katsanos KH, Christodoulou DK. Pancreatic Involvement in Inflammatory Bowel Disease: A Review. J Clin Med Res. 2018;10(10):743-751.
11. Crohns and Colitis Foundation (n.d). Signs and Symptoms of Ulcerative Colitis. Retrieved from https://www.crohnscolitisfoundation.org/what-is-ulcerative-colitis/symptoms
12. Freeman HJ. Iron deficiency anemia in celiac disease. World J Gastroenterol. 2015 August 21; 21(31):9233-9238.
13. Zaidel O, Lin HC. Uninvited Guests: The Impact of Small Intestinal Bacterial Overgrowth on Nutritional Status. Practical Gastroenterology. 2013; Nutrition Issues in Gastroenterology, Series #7: 23-34.
14. Struyvenberg MR, Martin CR, Freedman SD. Practical guide to exocrine pancreatic insufficiency – Breaking the myths. BMC Med. 2017; 15(1): 29.
15. Al-Kaade S (2020, February 3). What causes anemia in exocrine pancreatic insufficiency (EPI)? Medscape. Retrieved from https://www.medscape.com/answers/2121028-18736/what-causes-anemia-in-exocrinepancreatic-insufficiency-epi
16. Vujasinovic M, Valente R, Thorell A, Rutkowski W, Haas SL, Arnelo U, Martin L, Löhr JM. Pancreatic Exocrine Insufficiency after Bariatric Surgery. Nutrients. 2017 Nov 13;9(11):1241.
17. Uribarri-Gonzalez L, Nieto-García L, Martis-Sueiro A, Dominguez-Muñoz JE. Exocrine pancreatic function and dynamic of digestion after restrictive and malabsorptive bariatric surgery: a prospective, cross-sectional, and comparative study. Surg Obes Relat Dis. 2021 Oct;17(10):1766-1772.
18. The National Pancreas Foundation (n.d.). Exocrine Pancreatic Insufficiency (EPI). Retrieved from https://pancreasfoundation.org/patient-information/ailments-pancreas/exocrine-pancreatic-insufficiency-epi/
19. Cystic Fibrosis Foundation. (n.d.). Phthalates. Retrieved from https://www.cff.org/phthalates.
20. CREON® Full Prescribing Information. Chicago, IL: Abvie, Inc; 2023.
21. PERTZYE® Full Prescribing Information. Bethlehem, PA: Digestive Care, Inc; 2022.
22. VIOKACE™ Full Prescribing Information. Birmingham, AL: Allergan USA, Inc; 2012.
23. ZENPEP® Full Prescribing Information. Bridgewater, NJ: Aimmune Therapeutics, Inc; 2023.
24. Borowitz DS, et al. the Consensus Committee. Use of pancreatic enzyme supplements for patients with cystic fibrosis in the context of fibrosing colonopathy. J Pediatr. 1995; 127:681-84.